KingFisher Flex Phospho-enrichment protocol (using PureCube or IMAC beads)
PureCube beads prep
PureCube Fe-NTA MagBeads are delivered as a 25% suspension and are ready to use for phosphopeptide enrichment. Before use, dilute beads to 5% and wash three times with 80% ACN, 0.1% TFA. For all further handling steps beads are kept at 1 ml aliquots at a working concentration of 5%. Beads amount added to digested protein lysate is 2.5 uL/10 ug peptide.
Some examples:
5% PureCube Beads (uL) | Digested protein lysate (ug) |
|---|---|
| 250 | 1000 |
| 125 | 500 |
| 62.5 | 250 |
| 31.25 | 125 |
IMAC beads prep
IMAC beads are delivered as a 5% suspension. It requires steps to strip metal with 50 mM EDTA pH 8.0 as followings.
- Take a 1 mL aliquot of the 5% IMAC resin suspension (magnetic beads).
- Wash the aliquot of beads with 1 mL of water. (3 times wash)
- Incubate the IMAC resin in 1 mL of metal stripping solution (50 mM EDTA pH 8.0) for 30 min with shaking at room temperature. This step strips chelated metal ions.
- Wash the IMAC resin three times with 1 mL of water.
- Incubate the IMAC resin in 1 mL of metal loading solution (50 mM FeCl3) for 30 min with shaking at room temperature. This step chelates iron to the resin.
- Wash the IMAC resin three times in 1 mL of phosphopeptide binding solution (0.1% TFA in 80% Acetonitrile).
- Re-suspend the IMAC resin in 1 mL of phosphopeptide binding solution to create a 5% phosphopeptide enrichment. This prepared IMAC resin can be stored for several weeks at 4C until used for phosphopeptide enrichment.
The amount of the 5% IMAC suspension used for KingFisher Flex phosphor-enrichment is 75 ul for up to 1mg digested protein lysate.
Elution buffer (50:50 Acetonitrile: (1:20) ammonium hydroxide/water) prep
Add 25 ul of ammonium hydroxide and 475 ul of water to 500 ul acetonitrile.
10% FA in 75% ACN prep
Add 100 ul of FA and 150 ul of water to 750 ul of acetonitrile.
In-solution protein digestion can use either 6M Guanidine Hydrochloride, 8M Urea, or 4% Sodium Deoxycholate. Here I use 6M Guanidine Hydrochloride protocol as an example.
Hek 293T cell Trypsin in-solution digestion
- Lysed Hek Cell (cell lysed in 6M Guanidine Hydrochloride in 100 mM Tris-HCl pH8.0 followed by heating at 95C for 5 min., determine the total protein concentration by Bradford assay.
- Aliquot 1 mg of the above protein sample.
- Reduce disulfide bonds and carbamidomethylate cysteine residues by adding a 1:10 volume of reduction/alkylation buffer (100 mM TCEP and 400 mM 2-chloroacetamide) to the samples. Incubate samples for 5 min at 45 C with shaking (1,500 rpm).
- Diluted (DF=3) above samples with 100 mM Tris-HCl pH8.
- Add 10 ul of LysC (2 ug/ul) and 40 ul of Trypsin (0.5 ug/ul) at an enzyme-to-substrate ratio of 1:100 (wt/wt) (e.g., 2 ug of each enzyme for 200 ug of protein), reseal, and digest overnight at 37C with shaking (1,500 rpm).
Desalting
- After digestion, add 10% TFA to bring pH down to 2-3. Check the pH with pH paper and adjust accordingly.
- Spin down any insoluble material with 5 minutes, max speed (4000 rpm), room temp spin.
- Set up Sep Pak tC18 cartridges on a vacuum manifold. Use 1 cartridge per 5 mg of lysate.
- Activate column with 1 mL 80% ACN / 0.1% TFA.
- Equilibrate column with 3x 1 mL 0.1% TFA.
- Add sample to the column: samples were added 1 mL at a time, which is a little slow but prevents losses.
- Wash with 4 times with 1 mL 0.1% TFA.
- Place a labeled collection tube (15 mL conical) under the sample.
- Elute with 3x0.4 mL 50% ACN, 0.25% formic acid, 1.2 mL total.
- The sample was dried by SpeedVac overnight.
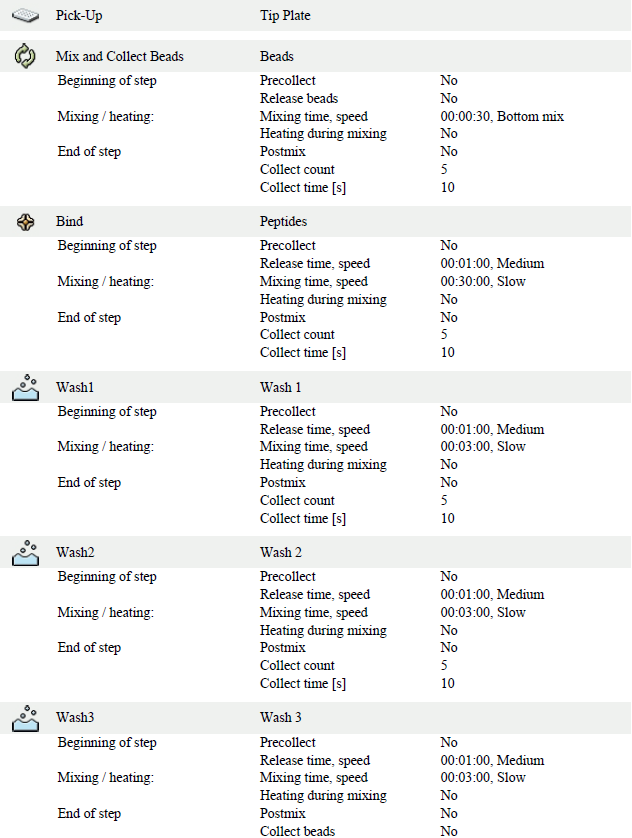
KingFiser Flex phosphoenrichment preparation
- Beads plate: Aliquot proper amount of beads (PureCure 250 ul, IMAC 75 ul) to the corresponding position of the standard plate
- Peptide plate (Standard 96 well plate): Resuspend peptide samples in 0.1% TFA in 80% ACN. Aliquot appropriate amount of peptide to the corresponding position of the standard plate.
- Three wash plates (Standard 96 well plate): Aliquot 150 ul of 0.1% TFA in 80% ACN to the corresponding positions.
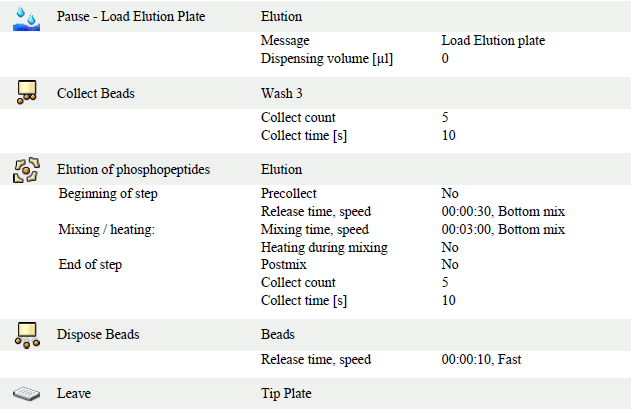
- Elution plate: Freshly prepare elution buffer (50:50 Acetonitrile: (1:20) ammonium hydroxide/water). Add 50 ul of the elution buffer to the corresponding position to the standard 96 well plate when the KingFisherFlex paused.
- BindIt program used at KingFisherFlex: IMAC_20200303.bdz
- After the entire procedure is done, transfer the total amount of the sample from the elution plate to another labeled tube contained 30 ul of 10% FA in 75% ACN solution. Mixed well.
- Transfer the above peptide mix to a Nest C18 column under the sample column set. Label the final sample tube. Centrifuge with 3000 rpm for 1 min. Dried samples by Speed Vac.
KingFisher Flex procedures